Parasites in Spotted Seatrout
The Estuarine Finfish Research section of SCDNR, in conjunction with the Parasitology laboratory of the College of Charleston, are conducting a pilot study on the life cycles of three common parasites; Kudoa inornata, Cardicola laruei, and Henneguya cynoscioni, which infect spotted seatrout in all South Carolina estuarine systems. Each parasite can be identified microscopically in fish tissue: K. inornata in the skeletal muscle, C. laruei in the heart ventricle, and H. cynoscioni in the bulbous arteriosus. Although these parasites do not infect humans, evidence shows that their presence impacts the health of their fish hosts. We used hatchery reared spotted seatrout to understand the life cycle of these parasites and how they infect wild seatrout.
Many parasite life cyclesinvolve two hosts: a fish and an aquatic invertebrate. As spores multiply in an infected fish, they are released and taken up by an intermediate host. It is believed that production of spores within the intermediate host results in the formation of a distinctly different type of spore, which is then transmitted back to the fish population. While the life cycles of these parasites are unknown, we can assume that they are similar to those described for closely related species that infect fishes. Transmission studies have been used to identify the life-cycles of similar freshwater parasites which have identified some types of freshwater worms (oligochaetes) as intermediate hosts. Because these types of worms are uncommon in marine environments, it is thought that a similar type of marine worm (polychaete annelids) isthe most likely candidate to be the invertebrate hosts in marine fishes. To date, only four marine Myxozoan life-cycles involving polychaete hosts have been defined. The objective of this pilot study is to identify the intermediate host of each parasite species of interest as well as the spores they produce.
For both the preliminary study and the current study, adult spotted seatrout broodstock were maintained at SCDNR’s Marine Resources Research Institute (MRRI) in disease-free closed recirculating aquaculture systems. Spawns were transported to the Waddell Mariculture Center (WMC) in Bluffton, South Carolina and stocked into ponds lined with high-density polyethylene liners and supplied with raw seawater pumped from the nearby Colleton River in the Port Royal Sound estuarine system. Approximately 30 days after stocking, ponds were drained and small juveniles were transported back to the MRRI laboratory for study. Juvenile seatrout were divided among six indoor tanks with a constant flow of settled seawater that was free of particulate matter and four outdoor tanks with a constant flow of raw unsettled seawater pumped directly from the Charleston Harbor. By considering the lineage of our juvenile spotted seatrout and tracking their transport history, it was possible to determine at which point in their life and under what conditions fish become infected.
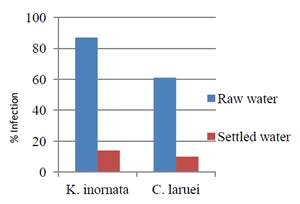
The preliminary study examined hatchery-reared spotted seatrout that had lived in raw seawater or settled seawater at the WMC or at the MRRI. After a period of two months, results showed that of the fish that spent time outside in flow-through Charleston Harbor water, 87% tested positive for the muscle parasite and 61% tested positive for the heart parasite. Of the fish stocked inside only 14% tested positive for the muscle parasite and 10% tested positive for the heart parasite (fig. 1). All fish from both systems tested negative for H. cynoscioni. These results suggest that while transmission in the water may occur, being close to worms in unfiltered Charleston Harbor seawater increased the likelihood of parasite transmission. It was also evident that the life-cycle of H. cynoscioni either required more time for formation of spores or was not present in either system.
Presently, the goals of the study are to determine percent infection over time in both systems and the environmental factors required by each parasite for spore production and to identify the intermediate host (species of worm) unique to each parasite. Toward this end, two-month-old spotted seatrout were harvested from ponds at the WMC and again distributed between the six indoor tanks and four outdoortanks as described in the preliminary study. Twenty fish were examined upon acclimation to determine if they were already infected by the parasites at the Waddell Mariculture Center. Fish harvested directly from ponds at the WMC and transported for release as part of the stocking research showed no infection of any of the parasites. Every month, three fish from each tank will be examined to determine if infection has occurred. As needed, settled sediment from the outdoorsystem will be sieved to collect benthic invertebrates (worms) which are introduced to the system through the raw seawater and all specimens collected will be examined microscopically to identify spores. It may be possible to identify intermediate hosts by collecting suspect invertebrates and housing them with uninfected spotted seatrout.
By understanding how transmission between hosts occurs; in particular, how parasite free hatchery-raised spotted seatrout become infected by each parasite in the laboratory and determining how long parasite development takes in these fish, we can better understand the impact of these infections on the wild population.



